UCLA researcher’s team finds common blood pressure medications do not increase COVID-19 risk
Dr. Marc Suchard, UCLA FSPH professor of biostatistics, co-led international research team looking at two widely used types of blood pressure drugs.
Research published in the peer-reviewed journal The Lancet Digital Health and co-led by a UCLA Fielding School of Public Health faculty member has found that two widely used types of blood pressure drugs are not tied to an increased risk of COVID-19 infection or complications.
The international team co-led by Dr. Marc Suchard, UCLA Fielding School professor of biostatistics, found that there was no increased risk of COVID-19 diagnosis, hospitalization, or subsequent complications for users of either angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers (ARBs). The study, the world’s largest, examined a group of more than 1.1 million patients in the United States and Spain using antihypertensives, researchers said.
“People with hypertension have worse COVID-19 outcomes, and there remains speculation that some anti-hypertensive medications may be detrimental,” Suchard said. “The clear answer is that ACE inhibitors and ARBs pose no increased risk as compared to other treatments.”
This real-world evidence, generated through open-science approaches, support recent regulatory and clinical recommendations that patients should not discontinue ACE inhibitor or ARB therapy due to concerns of increased COVID-19 risk.
“Based on our results, if there is a risk difference, it's marginal and would be very challenging to further refine outside such a large-scale international study," Suchard said.
The team included more than 30 scholars from more than 20 different universities and research centers, including UCLA, Columbia University, the University of Oxford, Yale University, and the University of Dundee in the United Kingdom.
“By comparing people exposed to ACE inhibitor and ARBs against people taking other antihypertensives, either alone or in combination, using two methods across three database the study generated 1280 comparisons to assess the safety of these drugs, producing highly consistent results,” said co-author Dr. Daniel Morales, Wellcome Trust research fellow at the University of Dundee.
The work was conducted under the aegis of the of the Observational Health Data Services and Informatics (OHDSI) community. OHDSI is a global, multi-stakeholder, interdisciplinary collaborative which aims to bring out the value of health data through large-scale analytics and an open-science approach in order to generate the evidence that promotes better health decisions and better care.
“Open science and collaboration are tenets of the OHDSI community, and they were never more important than early in this pandemic, when we all knew very little about critical questions like antihypertensive risks around COVID-19,” said co-author Dr. George Hripcsak, chair of the Columbia Department of Biomedical Informatics, the coordinating center for OHDSI, and a member of the Columbia Data Science Institute. “Our community collaborated for years to develop the high-level analytics which set the course for these studies, and our belief in international collaboration through open science allowed us to generate this reliable, reproducible COVID patient data that can inform and support critical decision-making to this and other issues challenging our healthcare community.”
Methods:
The team did a systematic and comprehensive federated active-comparator cohort study facilitated by a common data model. The protocol for the International Covid-ACE Receptor Inhibition Utilization and Safety (ICARIUS) studies was drafted and carried out by an international team of clinical, academic, government, and industry stakeholders through the Observational Health Data Sciences and Informatics (OHDSI) network
Funding:
The study was funded by the Wellcome Trust, the UK National Institute for Health Research, the US National Institutes of Health, the US Department of Veterans Affairs, Janssen Research & Development, IQVIA, the South Korean Ministry of Health and Welfare Republic, the Australian National Health and Medical Research Council, and European Health Data and Evidence Network.
Citation:
Morales, Conover, You et al. (2020) Renin–angiotensin system blockers and susceptibility to COVID-19: an international, open science, cohort analysis. The Lancet Digital Health, DOI: https://doi.org/10.1016/S2589-7500(20)30289-2
The UCLA Fielding School of Public Health, founded in 1961, is dedicated to enhancing the public's health by conducting innovative research, training future leaders and health professionals from diverse backgrounds, translating research into policy and practice, and serving our local communities and the communities of the nation and the world. The school has 631 students from 26 nations engaged in carrying out the vision of building healthy futures in greater Los Angeles, California, the nation and the world.
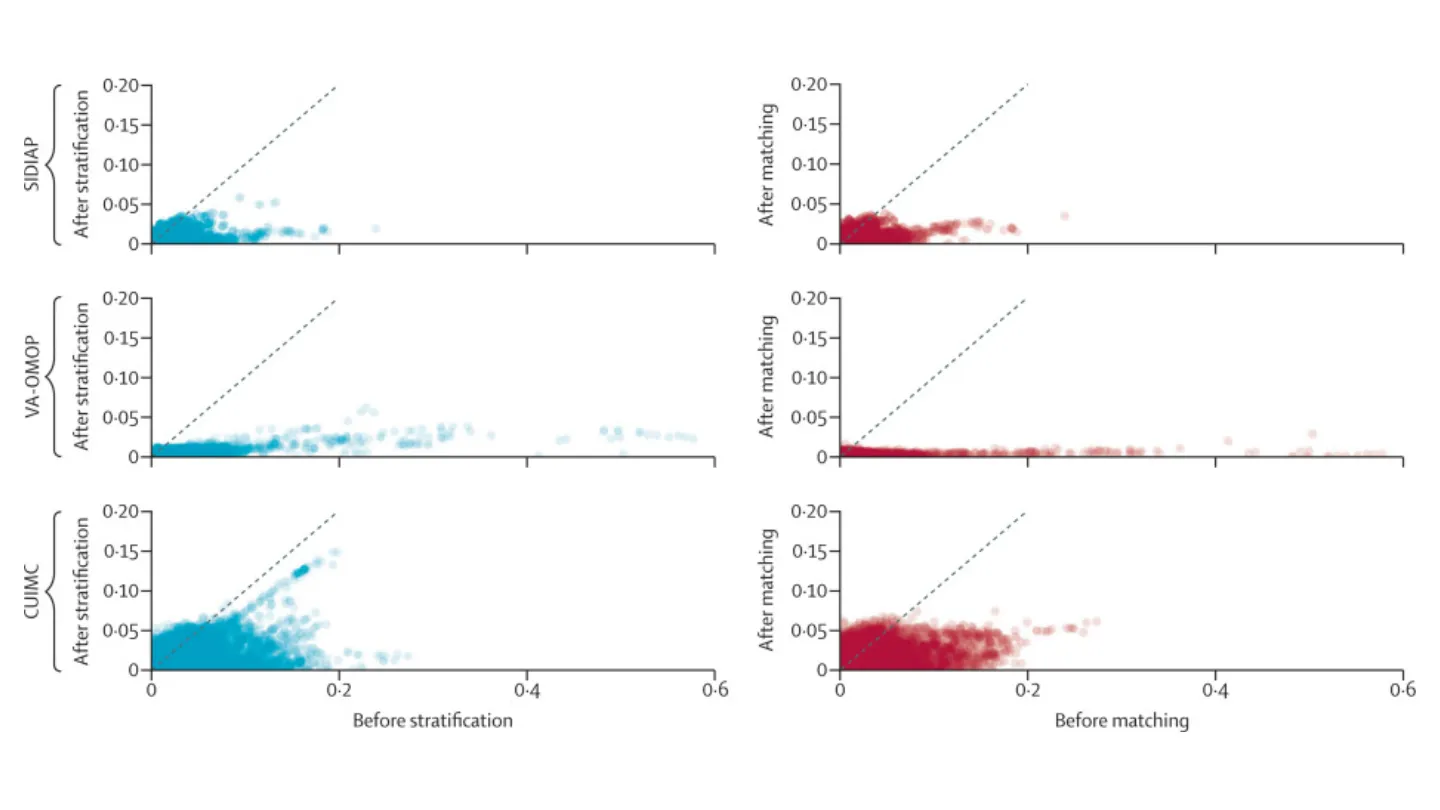
Cohort balance diagnostics comparing ACEI or ARB and CCB or THZ monotherapy prevalent use for the risk of COVID-19 diagnosis.
