Ebola vaccine being used in Congo produces lasting antibody response, study finds
UCLA-led study, led by Dr. Nicole Hoff and Dr. Anne Rimoin, finds evidence that antibody response and persistence after Ebola vaccination is robust.
UCLA Research Brief Findings
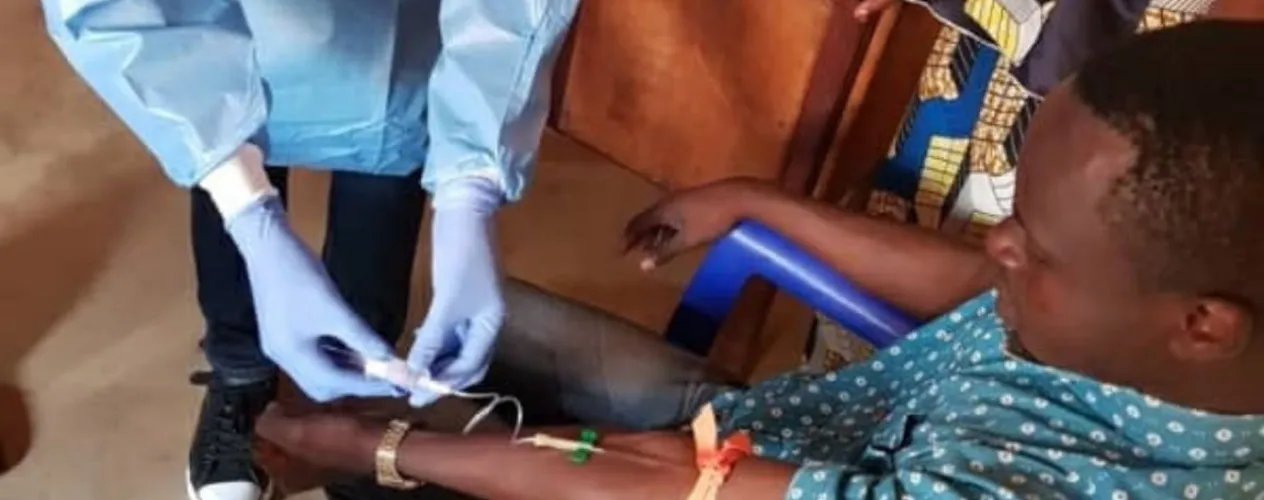
A new study by UCLA researchers and colleagues demonstrates that the Ebola vaccine known as rVSVΔG-ZEBOV-GP results in a robust and enduring antibody response among vaccinated individuals in areas of the Democratic Republic of Congo (DRC) that are experiencing outbreaks of the disease. Among the more than 600 study participants, 95.6% demonstrated antibody persistence six months after they received the vaccine.
The study is the first published research examining post–Ebola-vaccination antibody response in the DRC, a nation of nearly 90 million. While long-term analyses of the study cohort continue, the findings will help inform health officials’ approach to vaccine use for outbreak control, the researchers said.
Background
Ebola, one of the world’s deadliest viral diseases, was first identified in 1976 following an outbreak near the Ebola River in the DRC. Since then, outbreaks have occurred intermittently in sub-Saharan Africa, including 12 outbreaks in the DRC, where the disease remains endemic.
The single-dose rVSVΔG-ZEBOV-GP vaccine, officially licensed in 2019 by both the U.S. Food and Drug Administration and the European Medicines Agency, was administered to more than 300,000 individuals in the DRC during outbreaks between 2018 and 2020. Until now, however, studies examining the antibody response of vaccinated Congolese populations were lacking.
Method
UCLA researchers and their colleagues from the DRC’s National Institute of Biomedical Research studied individuals who received the vaccine during an Ebola outbreak in the DRC’s North Kivu Province. Between August and September 2018, the team worked alongside the DRC Ministry of Health’s Expanded Program for Immunization and the World Health Organization to enroll and vaccinate 608 eligible individuals who were contacts of people infected with Ebola or contacts of those contacts — an approach known as “ring vaccination” — as well as health care and frontline workers in affected or potentially affected areas.
The researchers collected blood samples at the time of vaccination, 21 days later and again after six months. The found that after 21 days, 87.2% of the study participants showed an antibody response. After six months, 95.6% of all participants showed antibody persistence.
Impact
The study provides crucial evidence that antibody response and persistence after rVSVΔG-ZEBOV-GP vaccination is robust in outbreak settings in the DRC. The findings are an important contribution to researchers’ and public health officials’ understanding of the vaccine response, which will aid in the ongoing development of strategies for deploying vaccines to control future outbreaks of Ebola in areas of the DRC and beyond.
Authors
The study’s first author is Dr. Nicole Hoff, assistant professor of epidemiology at the UCLA Fielding School of Public Health and the Kinshasa-based country director for the UCLA–DRC Research Program at UCLA; the corresponding author is Dr. Anne Rimoin, the Gordon–Levin Professor of Infectious Diseases and Public Health at the Fielding School. Additional authors are Fielding School researchers Dr. Adva Gadoth and doctoral student Anna Bratcher (co-first author).
Journal
The study is published online today in the peer-reviewed journal Proceedings of the National Academy of Sciences.
Funding
The research was supported by a grant from the Bill and Melinda Gates Foundation and by the Faucett Catalyst Fund, an FDA Medical Countermeasures Initiative contract and the National Institute of Allergy and Infectious Diseases. The funding sources had no role in the writing of the manuscript or the decision for publication.
The UCLA Fielding School of Public Health, founded in 1961, is dedicated to enhancing the public's health by conducting innovative research, training future leaders and health professionals from diverse backgrounds, translating research into policy and practice, and serving our local communities and the communities of the nation and the world. The school has 761 students from 26 nations engaged in carrying out the vision of building healthy futures in greater Los Angeles, California, the nation and the world.
Faculty Referenced by this Article

Dr. Anne Rimoin is a Professor of Epidemiology and holds the Gordon–Levin Endowed Chair in Infectious Diseases and Public Health.

Robert J. Kim-Farley, MD, MPH, is a Professor-in-Residence with joint appointments in the Departments of Epidemiology and Community Health Sciences

Dr. Joseph Davey is an infectious disease epidemiologist with over 20 years' experience leading research on HIV/STI services for women and children.





















































